The Office of the Executive Vice President for Research assists scholars and students across the University in regulatory compliance. This page offers numerous guides and resources to staying compliant while conducting research.
Research Handbooks
Note: Research Handbooks are now available through LabArchives.
The research handbooks are located at the top of the left-hand panel of LabArchives in the EVPR Procedures and Policies notebook.
1. Navigate to the LabArchives login and sign in with your UNI and UNI password on the blue Columbia UNI login screen.
2. If prompted, authenticate with Duo multi-factor authentication.
3. If this is your first LabArchives login, you need to confirm your account details. Your name and email will be auto-suggested, you just need to complete your profile by selecting a role type (if you are an administrative officer, "Researcher" is recommended). You do not need to fill anything in for ORCID. Click Update.
4. At the top of the left-hand navigation panel, click Notebooks.

5. Next, click the top notebook: EVPR Procedures and Policies.
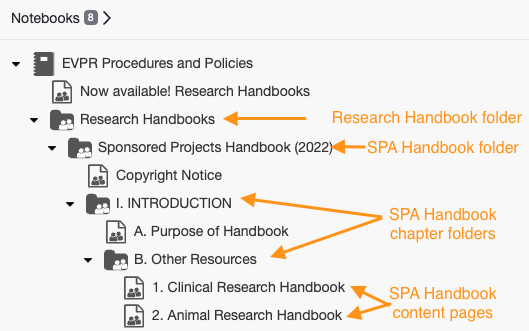
6. You can now use the left-hand navigation to display research handbook content. To view content:
- Select the Research Handbooks folder
- Select the handbook folder that you are interested in
- Select a chapter (section) folder to display the content pages below
- Click on a page to see the content on that specific topic
Gives practical guidance in the management of sponsored projects funded by both governmental and private organization, in accordance with the research policies and procedures of the University.
A companion resource to the Sponsored Projects Handbook, geared to the clinical research coordinator. Also includes a chapter on FDA-regulated research.
A companion resource to the Sponsored Projects Handbook, designed to be a reference guide for faculty and staff who are involved in research using animals.
Gives practical guidance to faculty, staff and students who are conducting research using hazardous materials.
Gives practical guidance on research using sources of ionizing radiation, in accordance with applicable laws, regulations and University policies and procedures.
Provides up to date, in-depth information on all policies, procedures and offerings for Columbia Postdocs.
FAQ
Select the role that seems most appropriate to you; if you are an administrative officer "Researcher" is recommended.
Your role selection is for user metrics only and will not affect anything you see in LabArchives.

It is strongly recommended to consult the "live" handbooks directly in LabArchives rather than relying on the point-in-time PDFs below. Although many revisions of the handbooks are made on an annual basis, changes can also be made at any time, and the PDFs below may not contain the most current information.
If you prefer to have a PDF version of the handbooks, PDF files of the research handbooks are available on LabArchives and will be from time-to-time as revisions are made, and at least on an annual basis.
Note: If you right-click on a notebook, you may see the "PDF" option. While this typically works for exporting LabArchives' notebooks, LabArchives has reported that it was not designed for notebooks that have been shared with the entire Columbia community and therefore it does not work for most users. We are working with LabArchives to improve the functionality.
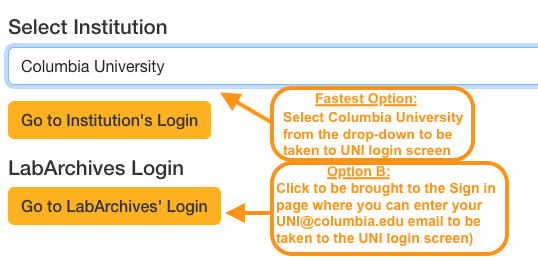
Both options work as long as you use your UNI!
- Fewest clicks: Choose Columbia University from the Select Institution drop-down to be taken directly to Columbia's standard navy UNI login screen.
- Also okay but extra clicks: Choose Go to LabArchives' Login, enter your [email protected] email or select Columbia University, and it will also take you to Columbia's standard navy UNI login screen.
Research Policies
Collection of policies often referred to by faculty and staff involved in research.
