Battery Safety
Lithium‐based batteries are integral to many of the world’s commonly used rechargeable electronic devices, from smartphones, laptops and tablets, to scooters, bikes and electric cars. Not only are they present in the general community, but they are also the focus of research activities in many laboratories and are present in some form in nearly all others. Many labs work with both commercial batteries and batteries fabricated in labs. Their research ranges from electrolyte characterization, energy storage, and non‐invasive battery failure determination. As lithium‐based batteries become more prevalent in all settings, the need to address their risks and how to use them safely increases.
Lithium‐based battery cells contain four main components: a cathode, an anode, a separator, and an electrolyte liquid (See figure 1). The cathode is the oxidizing electrode that acquires electrons from the circuit and is reduced during the reaction. The anode is the reducing electrode; it releases electrons to the external circuit and oxidizes during the reaction. The separator is a semipermeable membrane placed between the cathode and anode. The separator's main function is to keep the oppositely charged electrodes from interacting with each other and causing a short circuit. The electrolyte transports positively charged ions between the cathode and anode terminals. The solution is typically made of hydrocarbons and lithium‐based salts. The selection of electrolyte is integral to the performance and safety of the cell.
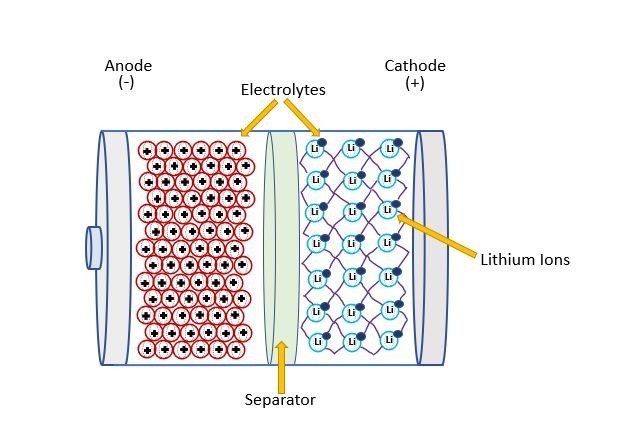
Figure 1: Lithium‐based battery cell diagram with four main components: a cathode, an anode, a separator, and an electrolyte liquid.
Thermal runaway occurs when heat generated from the battery escapes the cell. The separator melts and allows the anode and cathode to mix. When they react, they cause the temperature of the battery to dramatically increase. This phenomenon can ignite the flammable electrolyte solution and cause a fire or other potentially violent reactions. Below are some causes of thermal runaway:
- Internal short circuit caused by cells made with poor quality material.
- Overcharging of batteries.
- High or low temperature environments.
Commercial
- Lithium‐based batteries can store a large amount of energy in a small containment unit.
- Batteries are designed to be used in a specific manner, deviations from this can be dangerous.
- Like any consumer product, defects can be found in a small number of these batteries leading to overheating, fire, or explosion.
Lab‐fabricated
- Lab fabricated batteries face the same hazards as commercial batteries. However, since they are built in house for research, they may lack the safety tests usually done by battery companies.
- Potential exposure to the raw ingredients of a battery. (i.e., Lithium metal, electrolytes, etc.)
Follow manufacturers’ recommendations to ensure battery safety is prioritized.
Purchasing
Purchase batteries and battery‐powered devices from reputable manufacturers; high quality rechargeable devices can be easily distinguished by a UL (Underwriters Laboratories) or ETL (Electrical Testing Laboratories) label. This demarcation states the battery or device has been safety tested and will perform as expected compared to non‐UL or non‐ETL products.
Storage
Store batteries and battery‐powered devices at room temperature and out of direct sunlight. Store batteries and battery‐powered devices away from open flames and combustible materials. Do not store batteries in metal containers. Provide physical separation of batteries from conductive materials, including from metals or other batteries, as this can lead to a short circuit. Do not expose batteries to extreme temperatures. For those in research, consider storing your batteries in a fireproof casing when not in use or when in transport.
Charging
Charge batteries and battery‐containing devices only in accordance with manufacturers’ instructions. All chargers should bear the UL or ETL mark; never use third party chargers as they may cause the system to overheat because of overvoltage. Never leave batteries or devices charging unattended; ensure they are charging in a safe location (e.g., never charge on or near combustible materials). Never use charging as a storage method. Always allow batteries to cool before using, after charging and before charging, and after using.
Reminder! “Fleet charging” of multiple e‐scooter or e‐bike batteries is NOT permitted inside any Columbia University campus or residential building or on Columbia University property.
Transportation
Handle batteries with care when transporting and consider transporting in a heat‐resistant and fireproof case. Do not transport batteries in metal containers.
Please contact [email protected] for information about shipping batteries.
Discontinue use immediately if a battery or device appears to be overheating, bulging, changing color, leaking, or emitting a strange odor or sound. Many of these indicators are evidence of thermal runaway and it is important to be cautious as the gases produced may be flammable and toxic. If safe to do so, unplug and move the object away from anything combustible and call 911. If safe and comfortable to do so, use a CO2 (Class BC) or dry chemical (Class ABC) fire extinguisher to extinguish a manageable fire using the PASS method. Complete a Hazardous waste pick up request for burnt battery debris, leaking batteries, or compromised batteries. If the area is deemed unsafe, evacuate the area.
Commercial
- Do not discard Lithium based batteries in municipal trash.
- No metals drums should be used to store or waste batteries.
- Damaged, corroded, or disassembled batteries cannot be recycled and must be handled as hazardous waste.
- Undamaged small (< 2 lbs. in weight) batteries should be placed in one of the many specific batteries recycling bins found on campus (Scan QR code for locations).
- Prior to battery recycling, place clear tape on battery terminals to prevent contact with another terminal in order to prevent a short circuit.

Research/Lab Fabricated
- Follow hazardous waste procedures for disposal of fabricated batteries in research.
o Collect undamaged fabricated batteries, with taped terminals, in a waste container with an orange hazardous waste label.
o Coin/button cell batteries should be taped individually before placing them into a waste container.
o If the battery packs are custom made, please disassemble the segments.
o Complete the waste label entirely, list the electrolyte contained in the cell and any other constituents. - Damaged fabricated batteries are managed as hazardous waste and should be collected in a waste container with a filled orange label.
o If a fabricated battery is damaged, safely place the battery in a labeled waste container and move it under the exhaust.
o Anything with residual lithium (for example, disassembled battery parts) should be placed in mineral oil and collected as hazardous waste. - Do not store waste batteries in metal containers.

