Columbia University Biosafety Cabinet Resources
In accordance with Columbia University Policy and the Occupational Safety and Health Administration (OSHA) bloodborne pathogens standard, 29 CFR 1910.1030(e)(2) (iii)(B)] Biosafety Cabinets must be certified upon installation, relocation, and on an annual basis. This must be performed by an NSF Accredited Biosafety Cabinet Field Certifier to ensure the cabinet is functioning properly. A list of preferred vendors accredited to perform this service are available online.
Individual laboratories and departments are responsible for annual certifications of their biosafety cabinets. EH&S and Procurement Services are pleased to announce a change in Columbia’s UwPA suppliers for biological safety cabinet recertification and decontamination services. The change comes following the completion of a competitive RFP process conducted by EH&S and Procurement Services.
Companies now qualified by EH&S for this service are:
- Technical Safety Services (TSS)
ARC Supplier ID: 0000090337
(866)-536-5656
http://www.techsafety.com
Sales Representative: Nichole Stacey, (425) 780-0988, [email protected]
- Scientific Equipment Product Service (S.E.P.S.)
ARC Supplier ID: 000009735
(516)-997-9006
http://www.sepsservices.com
Sales Representatives: John Carroll, [email protected]. Joe Tantalo, [email protected]
- DigeLab Solutions
ARC Supplier ID: 0000158091
(201)-606-2225
http://www.digelabsolutions.com
Sales Representative: Beatriz Melgar, (201) 606-2225, [email protected]
ARC Contract Category Codes:
81101706 Laboratory Equipment Maintenance
81141504 Equipment Test Calibration or Repair
4110350201 Biosafety Cabinets—Minor
41103502 Fume Hoods—Minor
You are strongly encouraged to use a preferred supplier with a University-Wide Purchasing Agreement (UwPA), as they have already been approved by Columbia. Visit Learn about University-Wide Purchasing Agreements (UwPA) for more information. Using a non-approved supplier will require a variety of different documentation and methods of payment, and, in some cases, will require competitive bidding. Contact [email protected] if you wish to obtain information on other companies performing this service.
The renegotiated contracts have revised pricing and will offer cost savings to Departments and PIs. These suppliers have also agreed to a high standard of customer service related to convenient scheduling of visits, notification of expiring certifications and sharing of technical knowledge and best practices with investigators. The certification price is typically $125-150 if no other services are required.
We’d like to know how well these companies are performing. Your feedback on positive and negative experiences is valued. Please submit comments to [email protected].
More information:
https://www.finance.columbia.edu/content/laboratory-equipment-services (including ARC codes)
Baker will no longer be supporting certain Biological Safety Cabinet Models. Please see the notice from Baker regarding their obsolescence.
In accordance with the Occupational Safety and Health Administration (OSHA), workers must be trained in the use of biosafety cabinets. In accordance with Columbia University Policy, training must be refreshed every two years.
Biosafety cabinet (BSC) placement
The BSC should be located away from traffic patterns, doors, fans, ventilation registers, fume hoods, and any other air-handling device that could disrupt its air flow patterns. All windows in the room should be closed. The BSC should be located at the wall furthest from and facing the entry door. If this is not possible, the BSC should be located on the side wall perpendicular to the hinge side of the door.” For minimum clearances around the BSC, please refer to the NSF/ANSI 49 Annex I (in References).
Procedures and Practices for BSC Use
Always keep track of the magnehelic gauge position on the BSC as this can indicate a drop-in air pressure across the HEPA filter medium. If the reading fluctuates from the initial value following certification a vendor should be contacted to field test the cabinet. Note average gauge pressure is typically around 0.5” WC.

Prior to working in the BSC, allow the blower to run for at least 5 minutes to purge potential contaminants.
All materials not required for work should be removed. Overloading the cabinet or occluding the front and rear air intake grills of can impair the directional air flow.

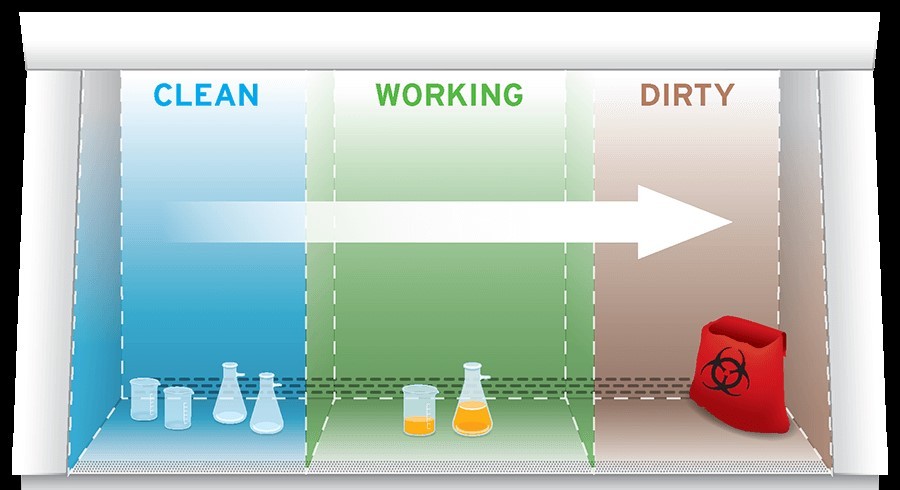
Adopting a work flow from clean to contaminated areas across the work surface will help mitigate cross contamination.
Biosafety Cabinet UV lamps
UV Lamps are often installed in BSC’s. The CDC, NIH, NSF and ABSA agree that UV lamps are neither recommended nor required in Biosafety Cabinets (BSC). While their germicidal capacity is often debated among users and manufacturers, UV lighting cannot be substituted for the routine use of a surface disinfectant with EPA registered claims. In order to remove dust and soiling lamps should be cleaned weekly while the UV light is turned OFF. UV lamps lose their intensity with time and their function must be assessed during certification to ensure it has not reached its lifespan. UV lamps often contain mercury and cannot be disposed of through the regular municipal waste stream. Contact EH&S for proper methods of UV lamp disposal.
Biosafety Cabinet relocation
BSC must be cleared by EH&s prior to a move/relocation. BSC relocating within a building must be disinfected with a fresh 10% bleach solution. BSC on castors may be moved carefully within a building without a clearance or subsequent recertification. BSC relocating to another building or campus must be decontaminated via gaseous sterilization by a University approved vendor before EH&S can issue a clearance for relocation and must be recertified upon relocation.
Cleaning and disinfection of BSC work area
Interior surfaces of the workspace should be disinfected with a compatible disinfectant for an appropriate contact time.” While 70% ethanol is an adequate disinfectant for some microorganisms such as lipid-enveloped viruses, it is not considered an appropriate disinfectant for non-enveloped viruses and other microorganisms such as fungal spores. Its rapid rate of evaporation also limits the effective contact time. Chlorine bleach is an oxidizing disinfectant with sporicidal activity. The CDC recommends a 10% bleach solution made fresh daily for regular surface decontamination and on small spills on noncritical surfaces. A best practice is to mark the date of receipt as bleach breaks down and loses its effectiveness after 6 months. Sodium hypochlorite is corrosive to stainless steel and must be wiped off with sterile water or 70%e ethanol, or neutralized with sodium thiosulfate, another disinfectant. Commercial products containing >0.5% hydrogen peroxide with EPA-registered claims as a hospital grade disinfectant are a non-corrosive option.

Disinfection beneath BSC work surfaces
Surface contaminants can be pulled into the air grilles underneath the work surface of the BSC. Such contamination of the BSC may potentially compromise the integrity and sterility of the user’s product. BSC vendors will professionally certify, or vapor decontaminate the cabinet, but they will not disinfect under the removable work surface tray. The Environmental Protection Agencies Standard Operating procedures for the use and maintenance of biological safety Cabinets recommend quarterly disinfection or cleaning of all contactable surfaces and removable parts of the BSC by the user. All removable parts and surfaces should be cleaned with a disinfectant that matches the biological agents being used in the BSC. If 10% bleach is used, a subsequent wiping with 70% ethanol will minimize bleach-related corrosion. Any contact times can be referenced from the EPA claims for validation against biological agents. The Biosafety Office can also be contacted at [email protected] for any inquiries regarding any EPA-registered disinfectant claims.

Decontamination of liquid cultures and media
For the decontamination of liquid culture, a fresh bleach solution will decolorize phenol red, a constituent of tissue culture media, ensuring proper decontamination. A pink flask color will indicate that the stock solution has either degraded or enough bleach has not been added to the collection flask.

Biosafety in Microbiology and Biomedical Laboratories-6th Edition Online addition of the CDC-NIH’s standard reference on Biological Safety (November, 2020)
OSHA's Bloodborne Pathogen Standard 29CFR-1910.1030 listed here for your viewing pleasure.
Biosafety Related SDS's Safety Data Sheets (SDS) for chemical products have been available to workers for many years. However because many laboratory workers, whether in research, public health, teaching, etc., are exposed to not only chemicals but infectious substances as well, there was a large gap in the readily available safety literature for employees. These SDS are produced for personnel working in the life sciences as quick safety reference material relating to infectious microorganisms.
The National Select Agents Registry Program The CDC and USDA requires that laboratories using or shipping certain toxins and high-hazard microorganisms obtain prior CDC/USDA approval for these activities. If you have any questions about compliance or applicability, contact EHS; a program is already in place to facilitate the approval process.
NIH Guidelines NIH guidelines that specify practices for constructing and handling: recombinant (DNA) molecule and organisms and viruses containing recombinant DNA molecules.
Principles of Laboratory Biosecurity Because of the growing concern about the possible use of biological, chemical, and radioactive materials as agents for terrorism, the CDC/NIH has added this section to the latest version of Biosafety in Microbiology and Biomedical Laboratories
Guide for the Care and Use of Laboratory Animals Online reference of the National Academy of Sciences (1996).
Institute of Comparative Medicine Homepage for the Columbia University Institute of Comparative Medicine. Check here for useful forms, training, and documents pertaining to the use of laboratory animals.
Animal housing requirements. Matrix that identifies the animal biosafety containment level for a number of different experimental models of infectious agent and viral vectors.
