Perchloric Acid
Perchloric acid is usually found as a colorless, odorless liquid. When chilled or diluted, perchloric acid can be used as an oxidizing agent. Interaction with organics, bases, and reduction agents may result in a fire or explosion as perchloric acid is heated. Perchloric acid becomes unstable and a strong oxidizer when heated to temperatures over 160oC. At concentrations higher than 70%, perchloric acid becomes unstable and is shock sensitive when dry.
According to Bretherick’s Handbook of Reactive Chemical Hazards “Most of the numerous and frequent hazards experienced with perchloric acid have been associated with either its exceptional oxidizing power or the inherent instability of its covalent compounds, some of which form readily.”
According NFPA 45 Chapter 7.12.1.2, “Perchloric acid shall be permitted to be used in a chemical fume hood that is not specifically designed for perchloric acid operations where the vapors are trapped and scrubbed before released into the hood.” Perchloric acid experiments in a standard chemical fume hood must be designed in such a way that perchloric acid vapors are trapped and scrubbed before being exhausted. Work with perchloric acid in concentrations up to 70%, at room temperature, must be done in a chemical fume hood that contains no organic chemicals or organic materials, dehydrating agents, or radioactive materials. The chemical fume hood must have a sign posted alerting lab personnel that perchloric acid work is being conducted in that hood.
Important Reminders
- Perchloric acid-specific hoods are currently only available at the Lamont Doherty Earth Observatory (LDEO) campus.
- Please contact EH&S if the laboratory is planning to use perchloric acid at concentrations greater than 70% or heating perchloric acid.
- Immediately contact EH&S if perchloric acid appears discolored.
- Do not mix perchloric acid with other chemicals or wastes, if possible.
- Perchloric acid >72% is forbidden for transportation per USDOT.
Perchloric acid is toxic and highly corrosive to all tissues and presents both acute and chronic health hazards. Acute effects may include, but are not limited to, severe burns on contact with the respiratory tract, mucous membranes, eyes, and skin. Prolonged exposure to perchloric acid may lead to bronchitis, angina, nosebleeds and/or perforation of the nasal septum, nasal congestion, teeth erosion, conjunctivitis, dermatitis, and ulceration of the skin. Skin contact can cause severe burns and irritation; eye contact can cause serious damage and irritation; harmful when swallowed. Pictograms include:

- Perchloric acid use and handling should occur only inside a functioning and EH&S-certified chemical fume hood designated for perchloric acid use. The sash of the fume hood should be kept at the lowest possible height that allows for safe handling of the reagents.
- Heating perchloric acid or use of anhydrous perchloric acid (>70%) must be done in a specialized perchloric acid fume hood equipped with a wash down system. These specialized fume hoods are currently only available at the Lamont Doherty Earth Observatory (LDEO) campus.
Contact EH&S at [email protected] for more information or for unique or specialty applications of perchloric acid.
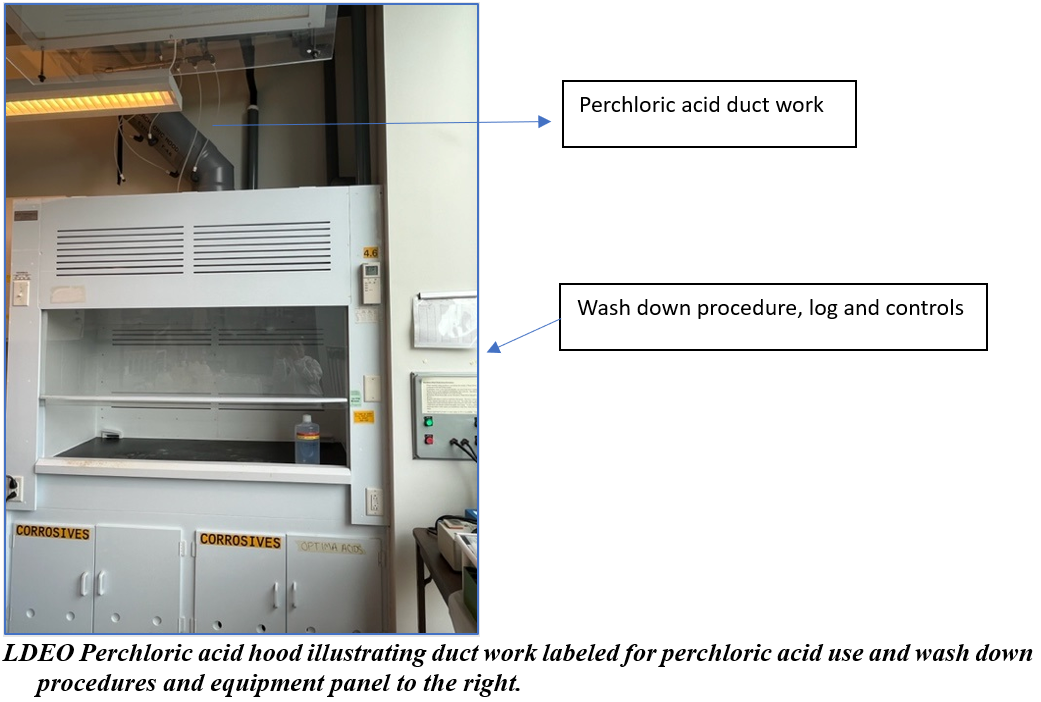
Please contact the EH&S office ([email protected]) about the use of perchloric acid. EH&S or the LDEO Safety Department will provide laboratories with perchloric acid spill kit materials. Contact [email protected] to obtain replacements and supply refills.
- Chemical waste disposal link for all campuses excluding LDEO
- EH&S Perchloric acid policy
- Perchloric acid FAQ Sheet
- LDEO Safety Office including campus specific waste pickup
- Standard Operating Procedure Template for Highly Hazardous Chemicals: Perchloric Acid
- EH&S provided perchloric acid spill kit contents
- EH&S provided perchloric acid spill directions
